Biotech startup closes $35 million financing round
Oct. 19, 2020
This paid piece is sponsored by South Dakota Biotech.
Alucent Biomedical Inc., which was founded by Avera Health to commercialize products using natural vascular scaffolding technology, has closed a $35 million Series B financing round led by a large multinational investor.
Even after Series B financing, Sioux Falls-based Alumend, a wholly owned subsidiary of Avera McKennan Hospital & University Health Center, remains the majority shareholder of Alucent.
“Anytime you can close that amount of investment from outside investors, it is a positive reinforcement of the technology,” said Ryan Hansen, Alumend’s president and CEO.
“The fact that we have two strategic partners really shows a firm validation of the technology and the potential opportunity. They see, as we believe, that this is a breakthrough technology that really will advance the vascular field for peripheral artery disease and other applications Alucent intends to develop.”
The financing round brings Alucent to $60 million in investment to date and includes a new investor, Fresenius Medical Care Ventures, an entity established to invest in startups and early-stage companies in the health care sector.
Alucent is a welcome addition to the portfolio, said Al Wiegman, head of Fresenius.
“The company’s first-rate management team is developing a revolutionary technology that has the potential to dramatically improve treatment for millions of people who suffer from PAD. We believe AlucentNVS stands to completely reshape how physicians think about treating vascular conditions,” Wiegman said.
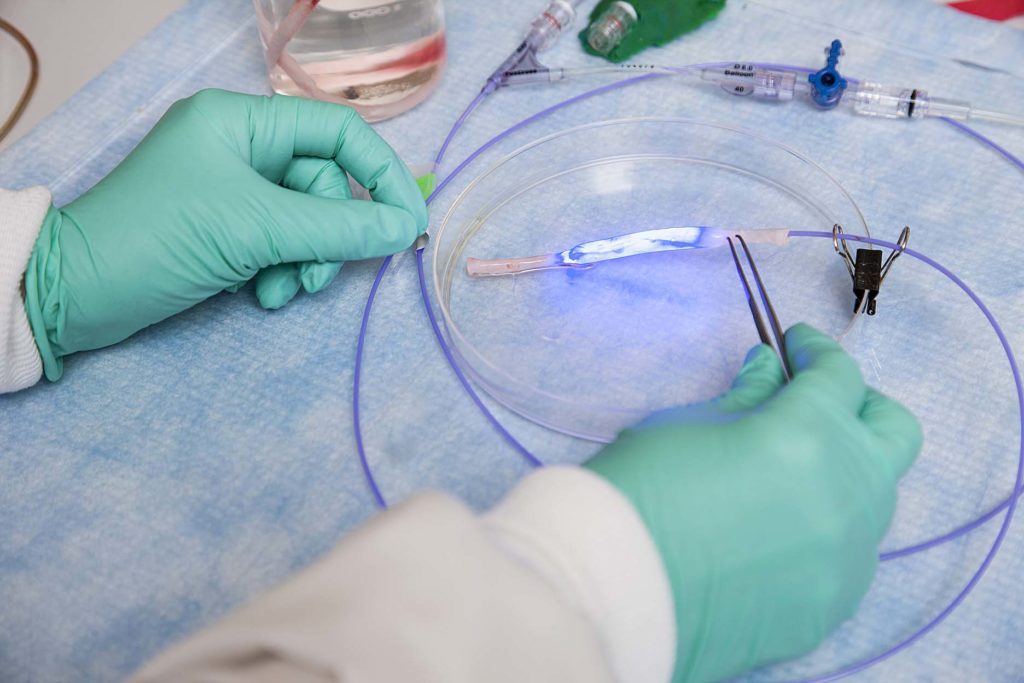
Natural Vascular Scaffolding, or NVS, involves using a revolutionary vessel-restoration system with photoactivated linking designed to treat peripheral artery disease, a painful and debilitating condition affecting more than 200 million people worldwide.
The NVS technology opens vessels and keeps them open without the use of permanent implants such as stents. Sustained, improved blood flow may result in pain relief, limb preservation, a reduction in reinterventions and an improved quality of life for patients.
Preclinical testing in animals has shown acute and long-term safety and patency without the pro-inflammatory and mechanical risks of placing a rigid foreign implant into the blood vessel, Alucent said.
The Series B funding will be used to support completion of phase one and two human trials evaluating the system’s safety and efficacy. Phase one has started and will include up to 15 participants across five U.S. sites. Phase two is planned for mid-2021 with up to 100 participants across 15 medical centers.
A larger phase three study will be needed before FDA approval to commercialize can be obtained, along with a reimbursement pathway for coverage, Hansen said.
“It’s a process, but it’s always a value-add when you have a strategic partner that can lend their industry knowledge and established infrastructure to advancing the technology,” he said.
Alumend also continues on the research and development side to work on new applications for the technology. Preclinical work is being done on a new application in the gastrointestinal field that would use the technology to target strictures, or narrowing, of the esophagus, which can cause difficult swallowing.
“Our preclinical data has shown the ability to significantly improve outcomes for a GI condition where there really are no existing applications that can sustain long-term positive results,” Hansen said. “We’re currently working on regulatory submission for approval to begin phase one testing.”
While Alucent is headquartered in Utah, the Alumend team continues to grow in Sioux Falls and is based at the USD GEAR Center. It also has added Dr. John Lee, Avera’s chief medical officer for cancer research, as medical liaison.
“As past chief medical officer for Immunity Bio Inc., he brings a lot of pharmaceutical industry experience and clinical expertise as a physician who has actually performed these GI procedures, so that adds a lot of value,” Hansen said.
“To have someone of his caliber in Sioux Falls can only expand and extend what we’re doing and keep the development of these companies here within the region. Our goal is to expand through success and additional financing and to do that in Sioux Falls as we look at our next ventures.”
Alumend and Alucent are perfect examples of how biotech companies are progressing in South Dakota, said Joni Johnson, executive director of South Dakota Biotech, the state’s industry trade association.
“Alumend actually began as a startup incubated in what is now Zeal Center for Entrepreneurship,” she said. “And thanks to partnering and relationships with our area health care field, higher education and now strategic investors, they’re positioned to commercialize and build on their core innovation. It’s exactly the sort of pathway we want to show exists for biotech startups in our state.”